Assessing the Clinical Effectiveness of Cologuard versus Colonoscopy in Early Detection of Colorectal Cancer
URS - Assessing the Clinical Effectiveness of Cologuard versus Colonoscopy in Early Detection of Colorectal Cancer
Project Info
2024 Aug – 2025 April, Undergraduate Research Society at University of South Florida
| 🔬 Team | Chandler Castillon, Lauren Song, Amanda Martin, Shriya Anand, Samia Khan, Riya Pandey |
Overview
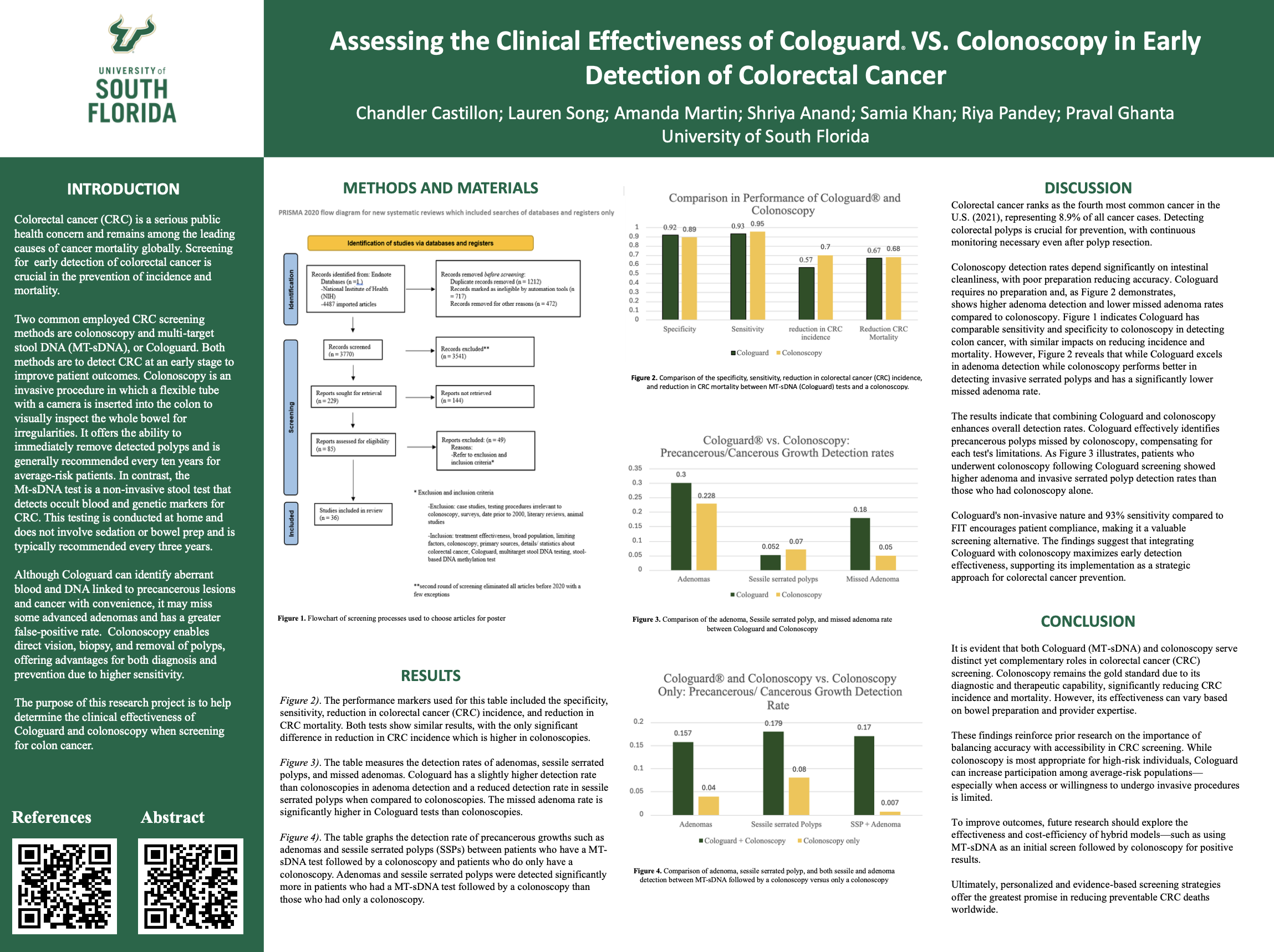
Welcome to my second research project! This research project compares two major colorectal cancer screening methods—Cologuard® (MT-sDNA) and colonoscopy—to determine which is more clinically effective in early detection. Our goal was to assess their respective strengths, limitations, and potential synergy in improving cancer prevention and outcomes.
Introduction
Colorectal cancer (CRC) is one of the leading causes of cancer-related deaths globally. Early detection is critical for improving survival rates, and two of the most widely used screening methods are colonoscopy and MT-sDNA testing (commercially known as Cologuard).
While colonoscopy is invasive and requires bowel prep and sedation, Cologuard is a non-invasive stool DNA test that can be done at home. Both have different strengths: colonoscopy enables immediate polyp removal, while Cologuard may encourage screening compliance due to its convenience.
Research Question
How does the clinical effectiveness of Cologuard compare to colonoscopy in early colorectal cancer detection?
We also explored:
- What are the relative strengths in detection rates for polyps and adenomas?
- Can combining both screening strategies lead to better outcomes?
- What are the implications for improving access and compliance?
Methodology
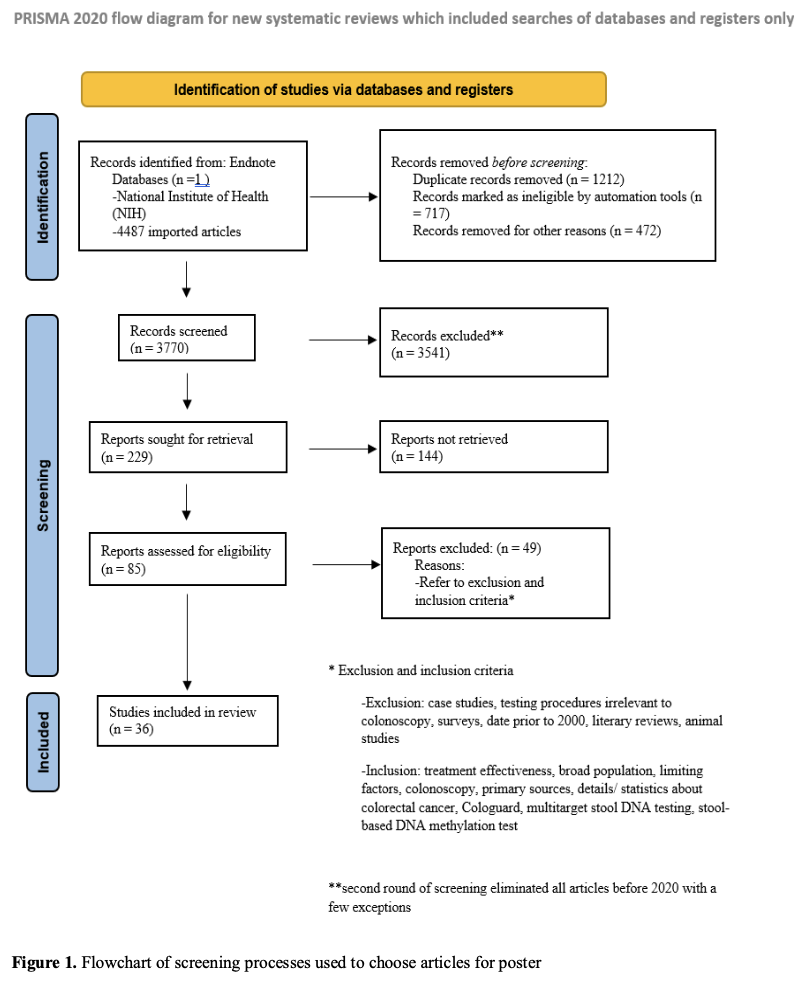
Data Collection
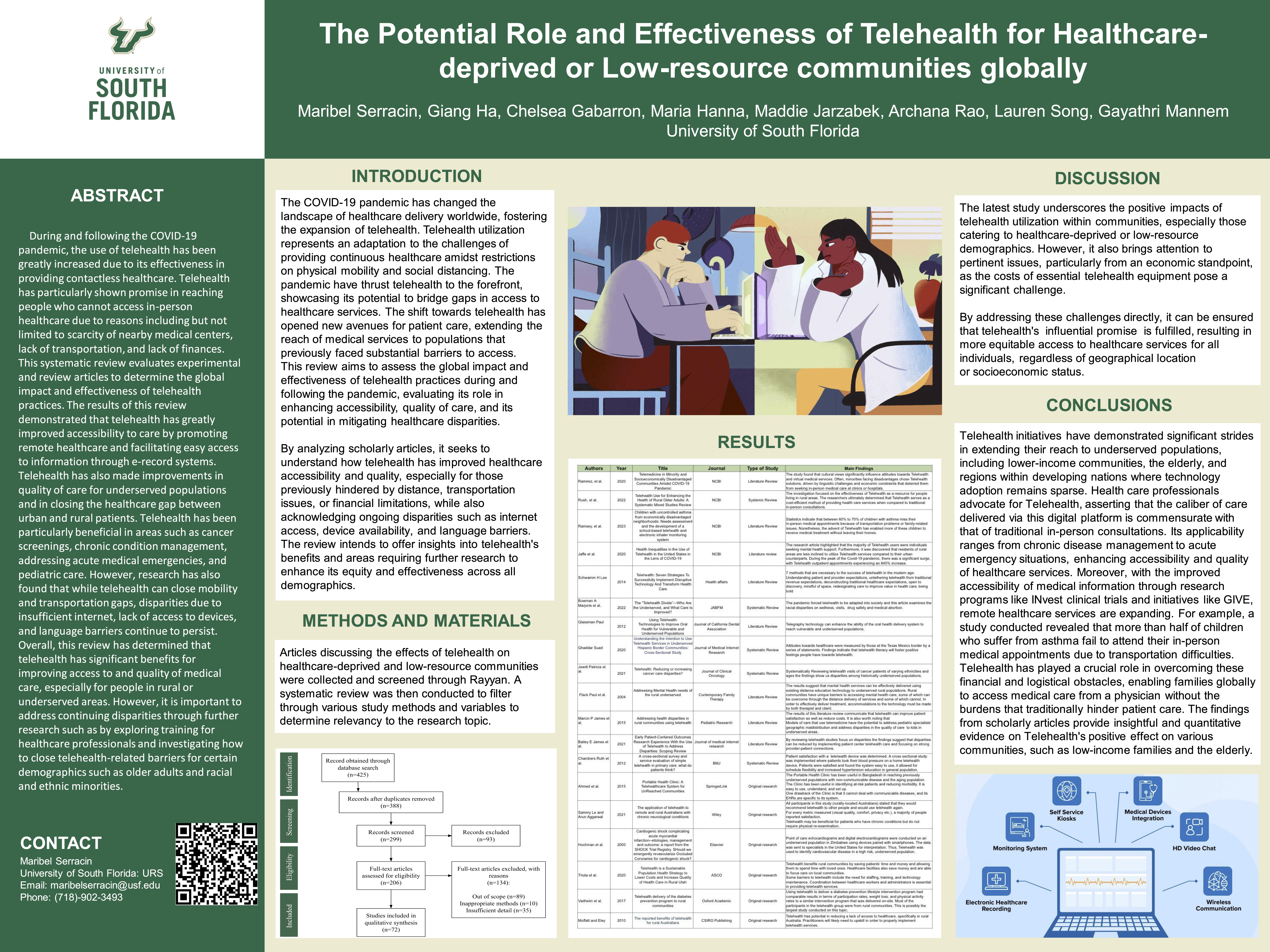
We reviewed published literature, including clinical trials and meta-analyses comparing sensitivity, specificity, and detection rates of both methods. Selection criteria were filtered through a defined flowchart (see Figure 1 of the poster).
Evaluation Criteria
Key clinical markers analyzed included:
- Sensitivity and specificity
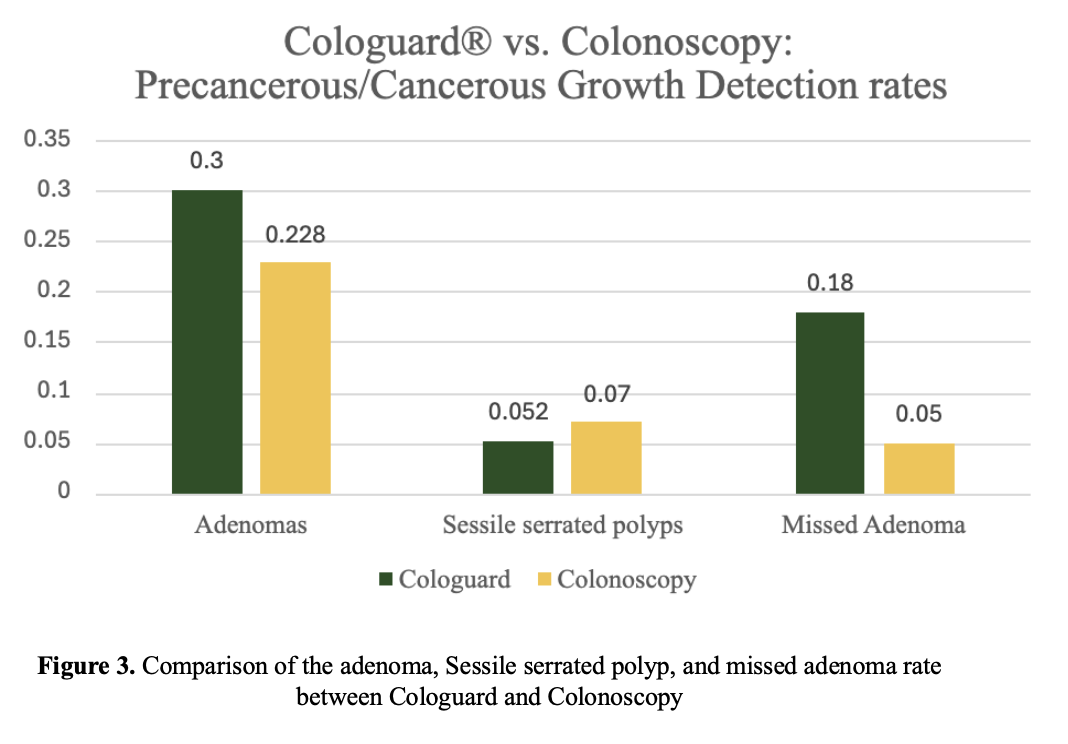
- Missed adenoma rates
- Detection rates for adenomas and sessile serrated polyps
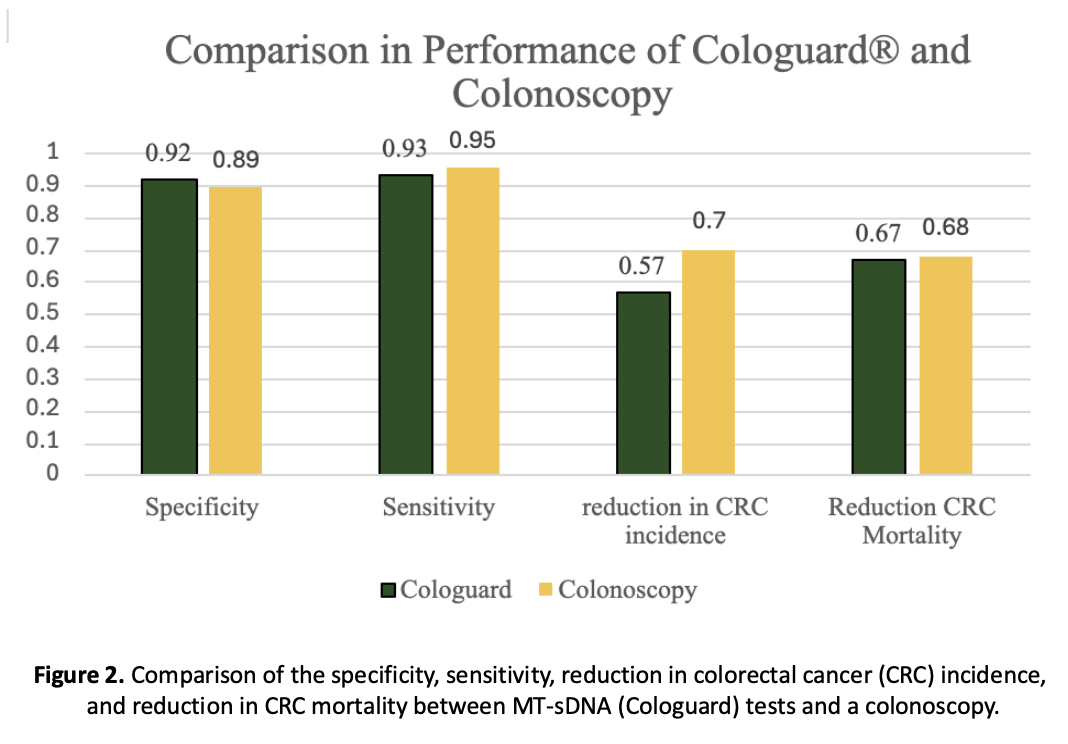
- Reduction in CRC incidence and mortality
Key Findings
-
Comparable Sensitivity
Both methods show similar performance in detecting colorectal cancer. Cologuard has a 93% sensitivity rate compared to FIT tests. -
Complementary Strengths
Colonoscopy outperforms in sessile serrated polyp detection and has a lower missed adenoma rate.
Cologuard has higher adenoma detection and no need for prep or sedation. -
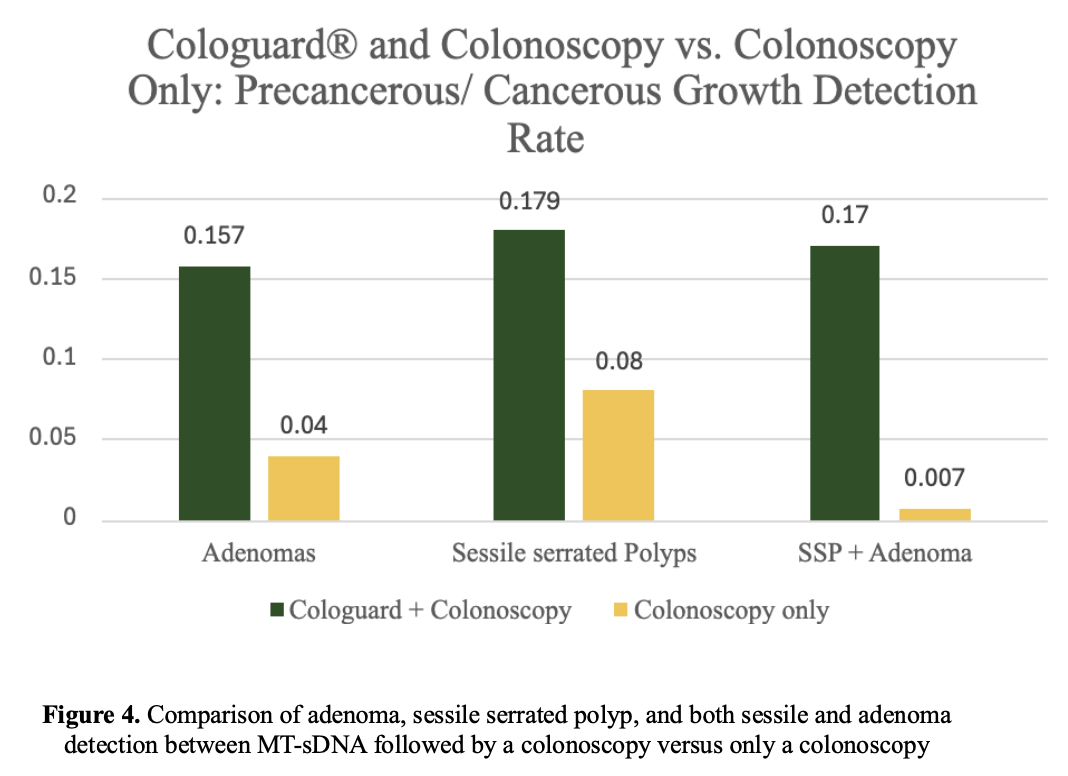
Improved Outcomes in Combination
Patients who underwent Cologuard followed by colonoscopy showed significantly higher detection rates than those who only had a colonoscopy.
Discussion
This study highlights the benefits of a hybrid screening strategy, particularly for average-risk populations. Cologuard can act as an initial, non-invasive screen, while colonoscopy provides diagnostic and therapeutic advantages. Together, they offer a more inclusive and effective model of early CRC detection.
Limitations
- Cologuard may miss some advanced adenomas and has higher false-positive rates
- Colonoscopy effectiveness varies based on provider skill and bowel prep quality
- Further research is needed on long-term cost-efficiency of hybrid screening models
Future Directions
- Evaluate real-world implementation of hybrid CRC screening programs
- Improve patient education and accessibility, especially for under-screened groups
- Investigate tech-driven screening enhancements (e.g., AI-supported diagnostics)
References
- [See all references and data sources on the original research poster]
- [Supplemental clinical trial reviews comparing MT-sDNA and colonoscopy]